Catalase-peroxidases (KatGs) are robust degraders of hydrogen peroxide. KatG figures prominently in the antioxidant defenses of several notorious pathogens, including Mycobacterium tuberculosis (cause of TB), Yersinia pestis (cause of bubonic plague), and Magnaporthe grisea (cause of rice blast disease and a major threat to world food security). This coincides with the fact peroxides are a central part of host defenses against pathogenic attack. Notably, in M. tuberculosis, KatG activates the antitubercular pro-drug isoniazid, one of the front-line agents used to fight TB. Consequently, mutations affecting the katG gene are a prominent underlying cause for resistance of numerous strains of Tb to isoniazid chemotherapy. Clearly, there are several biomedical benefits to be derived from understanding the connection between KatG structure and function.
The catalase activity of
catalase-peroxidases is an anomaly. KatG is a member of the same
superfamily as well-known peroxidase-only enzymes like
cytochrome c peroxidase (CcP). This is
immediately obvious when one compares the active sites of these
enzymes (see below). The active sites of KatG and
cytochrome c peroxidase, one of its closest relatives,
are superimposable. Despite the
great similarity of active sites across the superfamily, KatG
is the only one with appreciable catalase activity.
How is KatG able to do this? All
KatGs have a novel methionine-tyrosine-tryptophan (MYW) covalent
adduct. Substitutions to any member of the adduct wipe out the
catalase (but not peroxidase) activity of KatG. Thus, this MYW structure is a novel
protein-based cofactor that must give rise to unique mechanism
for degrading peroxide. This mechanism also includes a
conformationally dynamic arginine (the arginine switch) that
regulates electron transfer within the KatG protein, as well
as a substantial capacity to resist enzyme inactivation by
otherwise highly oxidizing intermediates. All of these
structural features combine to produce a hydrogen peroxide
decomposition catalyst ideal for resisting host innate immune
responses.
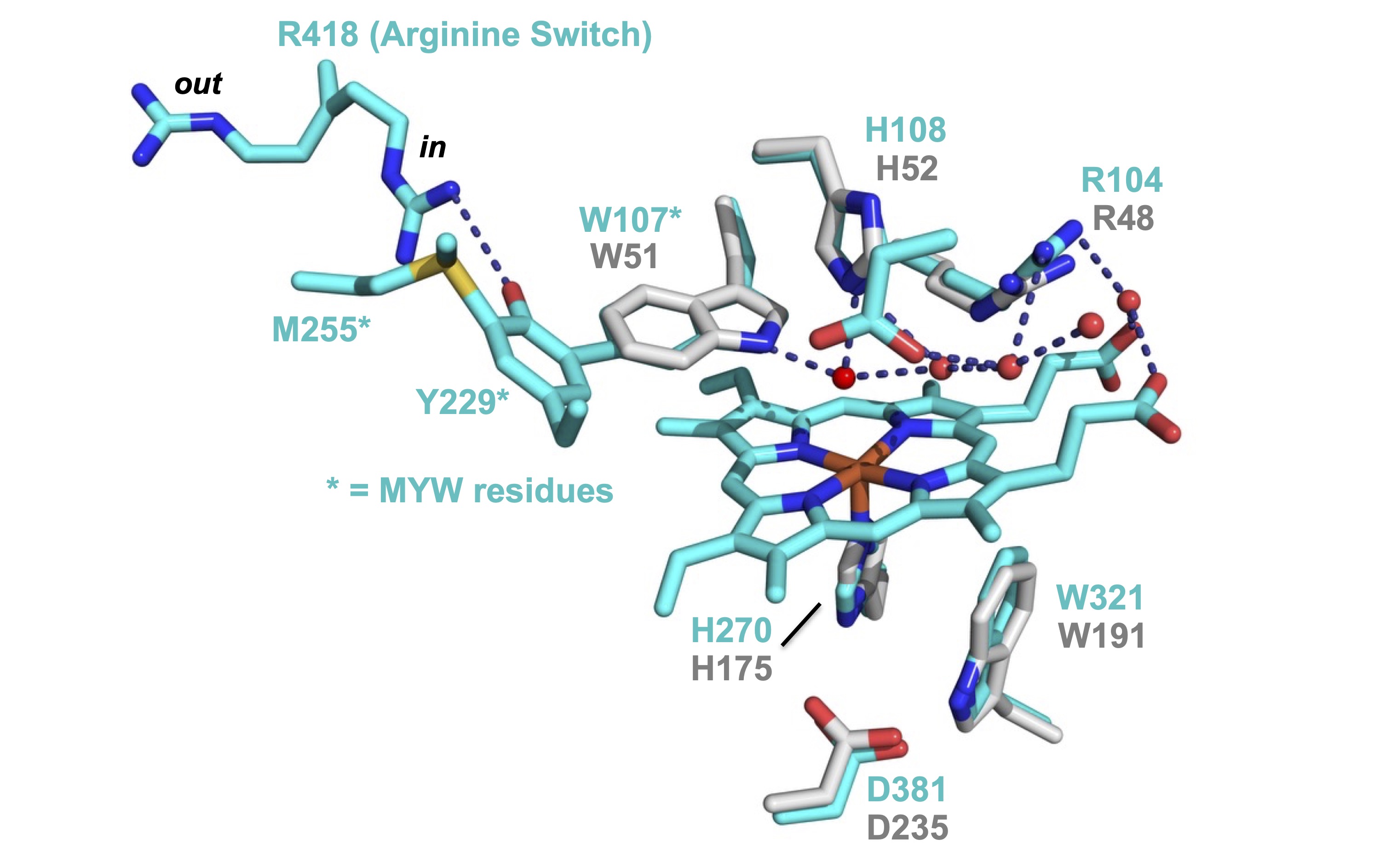
Image: KatG (cyan) and CcP (gray)
active sites superimposed on one another.