- 1. Most of the narcotic analgesics experience significant hepatic extraction and metabolism. This can be problematic in that many of the metabolites have longer half-lives than the parent drug as well as having CNS effects (e.g. excitation).
- a. Meperidine is metabolized to two metabolites, esterase hydrolysis to meperidinic acid (a rapidly eliminated metabolite) and CYP450 oxidation to normeperidine. Show the metabolic reactions involved in the formation of each of these metabolites. (Click here to see these reactions)
- b. The analgesic activity of meperidine may be reduced in patients receiving hydantoin anticonvulsant therapy. The hydantoins are well established CYP450 enzyme inducers. Explain the mechanism of this interaction between meperidine and the hydantoin anticonvulsants.
Induction (activation, increased levels) of CYP 450 enzymes responsible for the bioinactivation of meperidine by other drugs will result in a decrease in the pharmacologic and therapeutic effects of a usual therapeutic dose of the narcotic analgesic.
- b. The analgesic activity of meperidine may be reduced in patients receiving hydantoin anticonvulsant therapy. The hydantoins are well established CYP450 enzyme inducers. Explain the mechanism of this interaction between meperidine and the hydantoin anticonvulsants.
- 2. The dosage recommendations for parenteral sufentanil HCl suggest that an adjustment may be required in significantly obese patients. What is the basis for this recommendation?
Above average amounts of adipose tissue in a patient can serve as a site of loss for the highly lipid soluble sufentanil therefore an above average dose may be required in such patients to produce the desired level of analgesia.
- 3. In treating an overdose of a narcotic analgesic agonist with a narcotic analgesic antagonist it is important to know the elimination half-lives of both drugs. Explain why?
For optimal theapy, the duration of antagonist therapy must be similar to the duration of intoxication by the narcotic agonist. Hence, if an overdose involves an agonist with an appreciably longer half-life compared to the antagonist then additional dose(s) of the narcotic antagonist may be required to achieve full reversal.
- 4. A study has shown that administration of morphine oral solution following a high-fat meal increases the bioavailability of morphine by 34% compared to oral morphine administration in the fasting state. Explain the basis for these results.
Because of the relatively high lipid solubility of morphine, the presence of large amounts fat in the GIT significantly enhances the oral bioavailability this narcotic analgesic morphine by facilitating absorption from the appropriate site.
- 5. Two salt forms of the narcotic analgesic propoxyphene are marketed - the HCl salt and the napsylate salt (see structures below).
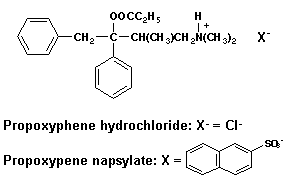
- 3. In treating an overdose of a narcotic analgesic agonist with a narcotic analgesic antagonist it is important to know the elimination half-lives of both drugs. Explain why?
- a. Given the usual dose of propoxyphene HCl of 65 mg. calculate the amount of napsylate salt required to provide an equianalgesic dose of the drug.
94.7 mg napsylate salt = 65 mg HCL salt realizing that each salt is intended to deliver a therapeutic amount of propoxyphene and taking into account differences in m.wt. of each salt form. Marketed dosage form actually contains 100 mg of the napsylate salt.
- b. Propoxyphene napsylate has much lower water solubility than the propoxyphene HCl salt. Explain how this might reduce the abuse potential of the napsylate salt.
Theoretically, the less water-soluble napsylate salt will be less likely abused as an I.V.-administered drug.
- b. Propoxyphene napsylate has much lower water solubility than the propoxyphene HCl salt. Explain how this might reduce the abuse potential of the napsylate salt.
- 6. Aqueous parenteral solutions of narcotic analgesics should not be mixed with either alkaline diluents (e.g. sodium bicarbonate solution) or solutions containing the sodium or potassium salts of other drugs. Explain why?
Because of their basicity, acid salts of narcotic analgesics are used to formulate aqueous parenteral solution. Admixture with an alkaline solution will result in an acid-base neutralization reaction occurring leading possibly to desolubilization of the narcotic analgesic.
NSAIDS
- 7. Salsalate is a salicylate prodrug converted metabolically to an active analgesic/NSAID.
- 7. Salsalate is a salicylate prodrug converted metabolically to an active analgesic/NSAID.
- a. Show the metabolic reactions and list the enzyme(s) involved in the bioactivation of salsalate.
Click here to see metabolic reactions. - b. Salsalate is a reversible cyclooxygenase inhibitor wheras aspirin exerts an irreversible inhibiton. What chemical difference(s) between the 2 drugs helps to explain this observation.
Salsalate is a prodrug of salicylic acid. Acetylsalicylic acid irreversibly inhibits cyclooxygenase because it contains an acetoxy ester function that can be transferred to the enzyme whereas salicylic acid, the active metabolite of salsalate has no such structural feature to react with the target enzyme.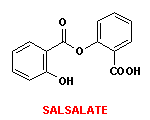
- b. Salsalate is a reversible cyclooxygenase inhibitor wheras aspirin exerts an irreversible inhibiton. What chemical difference(s) between the 2 drugs helps to explain this observation.
- 8. The oral bioavailability of certain NSAIDs is adversely affected by coadministration with aluminum and magnesium hydroxide containing antacids. Describe the chemistry of this interaction.
Certain NSAIDs may form insoluble salts/complexes with Al3+ and/or Mg2+ thereby lowering the oral bioavailability of these NSAIDs.
- 9. Alkalinization of the urine of NSAID-overdose patients may be of benefit in reducing toxicity. Explain the chemistry of the effect of urine alkalinization.
In an alkaline urine the acidic NSAIDs will be highly ionized and therefore less likely to be reabsorbed and more likely to be excreted thereby lowering tissue levels more rapidly.
- 10. The sulindac AUC may be increased in patients with hepatic disease (cirrhosis). Explain how this occurs and what therapeutic consequences it may have.
The therapeutic activity of sulindac is dependent on its hepatic bioactivation (reduction). Hepatic disease may reduce the efficiency of this organ leading to increased levels of the NSAID prodrug sulindac and reduced bioavailability of its sulfide metabolite, the active NSAID.
You can download a WordPerfect 6.1 (quiz2.wpd) file of this page by clicking here!
Click here to return to: PY420 Home Page
- 9. Alkalinization of the urine of NSAID-overdose patients may be of benefit in reducing toxicity. Explain the chemistry of the effect of urine alkalinization.