MED. CHEM. I - QUIZ # 3
PRACTICE
Answers are given in
- Answer the following questions about the drug structures shown here (click).
- 1. List a structural class for each of these drug structures (be as specific as possible). Click here for answers
- 2. List a therapeutic (not pharmacological) use for each of these drug structures. Click here for answers
- 3. List those drug structures that are acidic organic compounds (pKa 3-12). Click here for answers
- 4. List those drug structures that are basic organic compounds (pKa 3-12). Click here for answers
- 5. List those drug structures that are neutral organic compounds. Click here for answers
- 6. Describe the structural features of Drug #1 that make it a more useful therapeutic agent than ethanol (C2H5OH).
- Additional lipophilic hydrocarbon structural features (facilitate distribution to CNS site of action
- Tertiary alochol function - greater metabolic stability therefore clinically compatibile biodisposition profile.
- 7. The duration of therapeutic effects of drug #2 is much shorter than that of drug #4. Provide a plausible explanation for this difference.
- Drug #2, a thiobarbiturate derivative, is significantly more lipid soluble than drug #4, a barbiturate derivative. Rapid tissue redistribution will serve to shorten the pharmacological actions of drug #2 to a greater extent than drug #4.
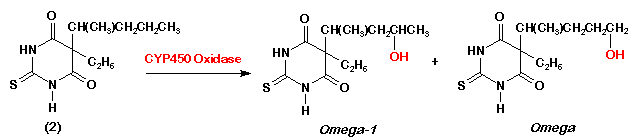
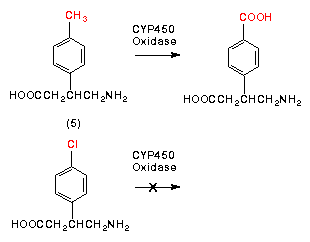
- 8. Draw the structure of the omega- and omega-1 CYP450 oxidation metabolites of drug #2.
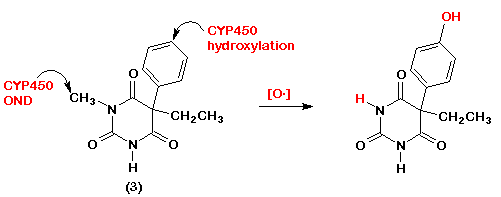
- 9. Discuss a plausible mechanism for the CNS depressant effects of drug #3. Show the structure of a water-soluble oral dosage form of drug #3.
- Since the stucture of drug #3 closely resembles that of valproic acid one can assume that the effects of drug #3 on GABA neurotransmission will be similar to those of valproate, i.e., conservation of the neurotransmitter by inhibiting the catabolic enzyme, GABA-T.
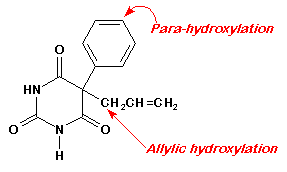
- 10. Draw the structures of the metabolites of drug #4 resulting from (i) aromatic hydroxylation and (ii) allylic oxidation.
- 11. Which drug, 5 or 6, will have the longer duration of therapeutic action? Explain why.
- Clearance of drug #6 by metabolism will require more extensive biotransformation because of its structural features (note N1-substituent) thereby providing it with a longer duration of therapeutic action.
- 12. Drug #5 degrades in acidic solution resulting in the formation of two products. Show these reactions and comment on their pharmaceutical signfiicance.
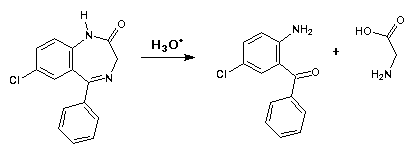
- Benzodiazepines cannot be formulated for parenteral administration in aqueous acid solutions since they degrade via hydrolysis of the 1,4-diazepine ring system. Parenteral formulations must be prepared utilizing nonaqueous solvents.
- 13. Draw the structure of a prodrug of drug 7 suitable for formulation in an aqeuous, well tolerated parenteral (IV and IM) dosage form.
Click here to return to: PY420 Home Page




