Maxwell's Demon


Maxwell's Demon is an imaginary creature that the mathematician James Clerk Maxwell created to contradict the second law of thermodynamics. Suppose that you have a box filled with a gas at some temperature. This means that the average speed of the molecules is a certain amount depending on the temperature. Some of the molecules will be going faster than average and some will be going slower than average. Suppose that a partition is placed across the middle of the box separating the two sides into left and right. Both sides of the box are now filled with the gas at the same temperature. Maxwell imagined a molecule sized trap door in the partition with his minuscule creature poised at the door who is observing the molecules. When a faster than average molecule approaches the door he makes certain that it ends up on the left side (by opening the tiny door if it's coming from the right) and when a slower than average molecule approaches the door he makes sure that it ends up on the right side. So after these operations he ends up with a box in which all the faster than average gas molecules are in the left side and all the slower than average ones are in the right side. So the box is hot on the left and cold on the right. Then one can use this separation of temperature to run a heat engine by allowing the heat to flow from the hot side to the cold side.
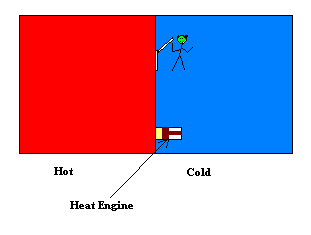
Another possible action of the demon is that he can observe the molecules and only open the door if a molecule is approaching the trap door from the right. This would result in all the molecules ending up on the left side. Again this setup can be used to run an engine. This time one could place a piston in the partition and allow the gas to flow into the piston chamber thereby pushing a rod and producing useful mechanical work.
This imaginary situation seemed to contradict the second law of thermodynamics. To explain the paradox scientists point out that to realize such a possibility the demon would still need to use energy to observe the molecules (in the form of photons for example). And the demon itself (plus the trap door mechanism) would gain entropy from the gas as it moved the trap door. Thus the total entropy of the system still increases.
The demon is trying to create more useful energy from the system than there was originally. Equivalently he was decreasing the randomness of the system (by ordering the molecules according to a certain rule) which is decreasing the entropy. No such violation of the second law of thermodynamics has ever been found.