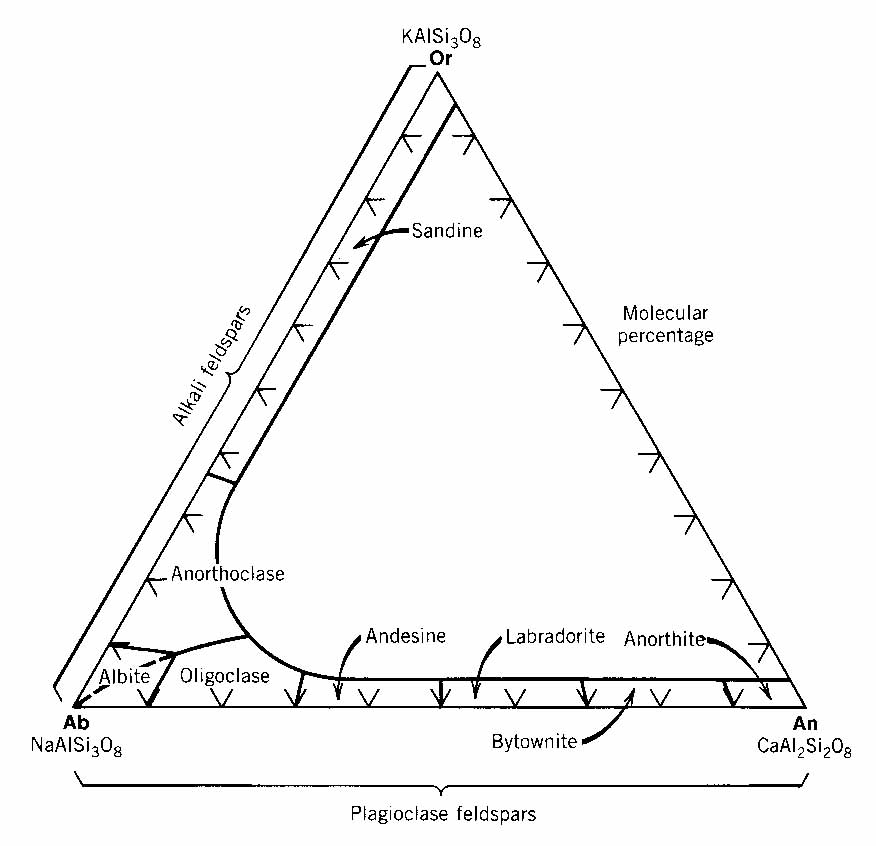
 Common
feldspars can be expressed in terms of the system
orthoclase - albite - anorthite. The members of the series orthoclase-albite
are known as the alkali feldspars,
and those in the series albite-anorthite are the plagioclase
feldspars.
Common
feldspars can be expressed in terms of the system
orthoclase - albite - anorthite. The members of the series orthoclase-albite
are known as the alkali feldspars,
and those in the series albite-anorthite are the plagioclase
feldspars.
(Further Development of this page is in progress)
Tectosilicates: Feldspars
Feldspars are the single most common group of minerals in Earth's crust. To put it into perspective, the total volume of plagioclase in the crust is greater than that of all the water in all the oceans! The Moon, also, has a crust composed mainly of feldspars - as the highlands (which appear white) are made of the plagioclase-rich rock 'anorthosite', and the Maria (darker, circular regions) are underlain by the plagioclase-rich rock 'basalt'. The very name 'feldspars' comes from Old German words meaning 'Field Stones', in reference to their abundance. Feldspars, along with quartz and the feldspathoids, are used as a basis for classifying and naming felsic igneous rocks (granites, rhyolites, etc.). The identity of feldspar polymorphs, composition, and textures can tell us a lot about the crustal setting in which a rock formed. Feldspars are important economic minerals, for the manufacture of porcelain, glass, and some ornamental stones.
 Common
feldspars can be expressed in terms of the system
orthoclase - albite - anorthite. The members of the series orthoclase-albite
are known as the alkali feldspars,
and those in the series albite-anorthite are the plagioclase
feldspars.
Common
feldspars can be expressed in terms of the system
orthoclase - albite - anorthite. The members of the series orthoclase-albite
are known as the alkali feldspars,
and those in the series albite-anorthite are the plagioclase
feldspars.
Felsspar structures can be described as an infinite network of SiO4 and AlO4 tetrahedra, which is a 'stuffed' derivative of the SiO2 structures, with substitution of Al for some Si into tetrahedral sites, and accomodation of Na, K, and Ca in voids. Na, K, Ca, and Al balance the substitutions for Si+4 as follows:
Si4O8 - Si+4
+ (NaAl)+4 = NaAlSi3O8
Si4O8 - Si+4
+ (NaAl)+4 = NaAlSi3O8
Si4O8 - 2Si+4
+ (CaAl2)+8
= CaAl2Si2O8.
It is particularly important to consider Al substitution into the tetrahedral sites of feldspars, because of the similar ionic radii of Al and Si, and the crustal abundance of Al; other trivalent cations such as chrome and ferric iron, can substitute into tetrahedral sites of feldspars. The larger voids in feldspar structures favor larger cations, such as Na, K, Ca, and sometimes Pb, Rb, Cs. Water can also be incorporated into feldspar structures. Although water, lead, and ferric iron are typically, at most, very minor constituents of feldspars, the color of feldspars is strongly influenced by these elements. For example, the alkali feldspars are commonly white or clear and colorless, but small amounts of ferric iron commonly give them a pink to red color, and small amounts of water and lead give them a blue to green color (as in the semiprecious stone amazonite).
| Sanidine | KAlSi3O8 | Monoclinic |
| Orthoclase | KAlSi3O8 | Monoclinic |
| Miocrocline | KAlSi3O8 | Triclinic |
You can think of the feldspar structure as having four-membered rings of (Si,Al)O4 tetrahedra linked into chains that parallel the a-axis, shaped like double-crankshafts, and the larger sites fitting into large spaces that alternate with the tetrahedra.
In the high-temperature polymorph of KAlSi3O8, Sanidine, Al and Si are completely disordered. That is to say, the occurrence of Al and Si in the tetrahedral sites of sanidine is random. This results in a higher symmetry of sanidine, as there are fewer 'special positions' that are restricted to either Al or Si (as in the more ordered polymorphs, orthoclase and microcline).
The alkali feldspar series (Albite to Orthoclase/Microcline/Sanidine) show complete solid solution only at high temperatures. For example, sanidite-high albite solid solutions exist at high temperature, but given time during cooling, the mixture will exsolve to form an interlocking network of low-albite and microcline Ñ 'perthite' Ñ at low temperature. In otherwords, there is ideal substitution of albite and potassium feldspar at high temepratures, but a miscibility gap and incomplete solid solution at lower temperatures.
Composition and structures of the feldspar minerals thus are often functions of cooling rate, and we can evaluate the thermal history of a rock by examining the feldspar textures.
Review Feldspar T-X diagrams.
| Albite | NaAlSi3O8 | Triclinic |
| Anorthite | CaAl2Si2O8 | Monoclinic |

In view of their importance and characteristic occurrences, compositions of the plagioclase solid solution series are subdivided, and named as follows: Albite (An0-10), Oligioclase(An10-30), Andesine(An30-50), Labradorite(An50-70), Bytownite(An70-90), and Anorthite(An90-100). (Find a way to remember this - such as, "All orangutans are laughable, baboonish animals".)